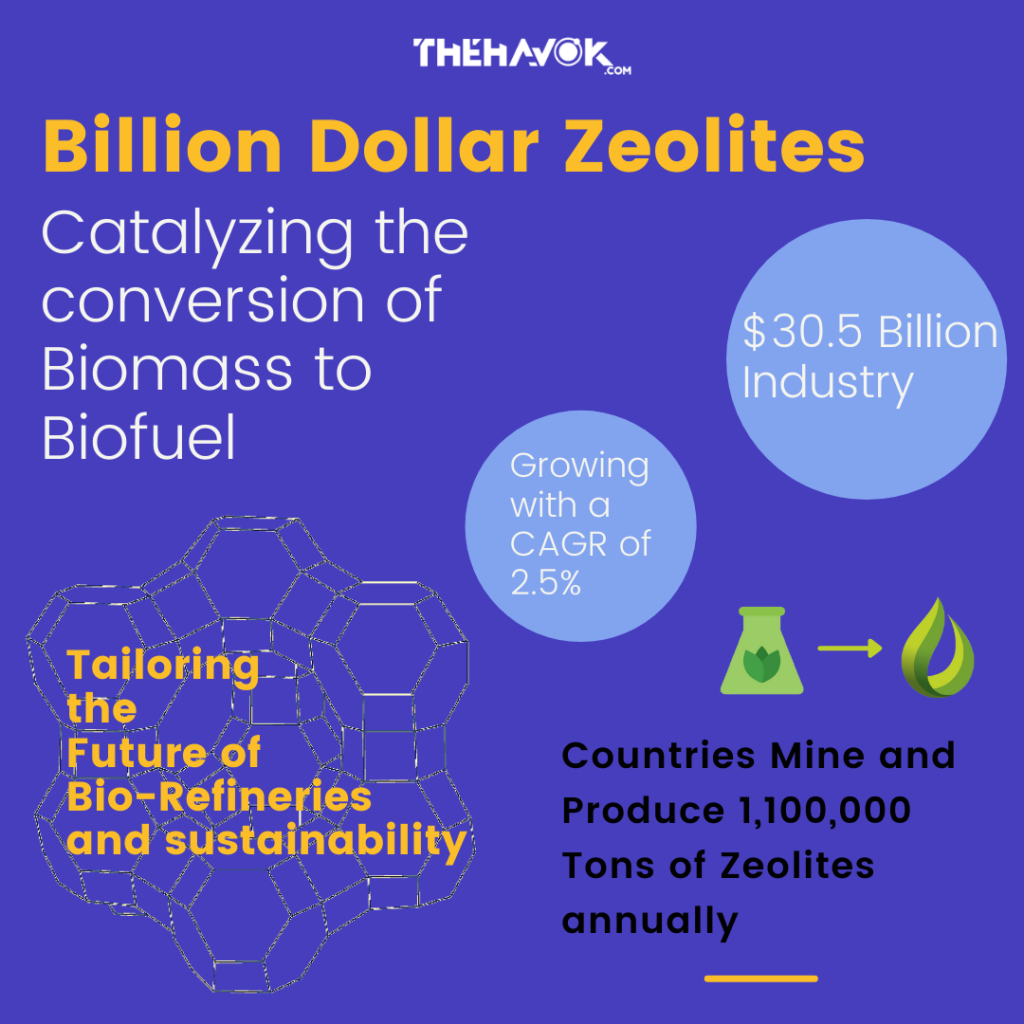
Zeolites. They are microporous crystalline solids mainly containing aluminum and silicon compounds. Used in construction and building materials, animal feed, agriculture, wastewater treatment, soil remediation and in other end-user products; the Zeolite industry had a market size of nearly 30.5 billion US dollars. Expected to grow with a compound annual growth rate (CAGR) of 2.5%; this market size is projected to hit 60 billion USD mark till 2027. Zeolite are extremely porous materials. They have a high internal surface area to size ratio. Interestingly, only 10 grams of it can have an internal surface area the size of a soccer field.
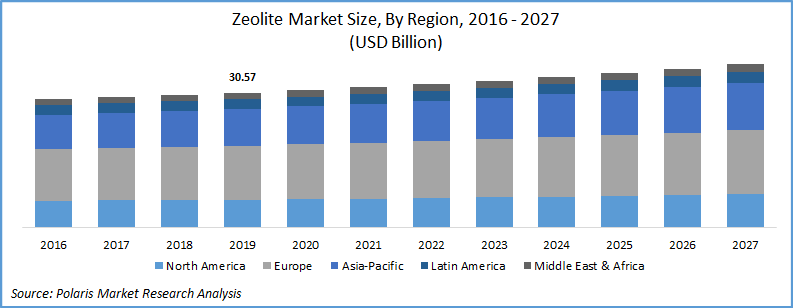
past and future growth
(Source: Polaris market research analysis)
Apart from such its intense use in cement, detergent and wastewater treatment industry; the cavities of zeolite are yet another property for which scientists are curious about. Zeolite cavities make them useful in catalyzing the chemical reactions and thus store and save energy in them. It is for the first time now, that a team of international researchers have made findings regarding the role of water molecules in these processes. So, let’s get a closer (rather microscopic) look at the role of water molecules to the reactions inside the zeolite’s pores; which are a few nanometers in size.
The Role of Acid
Acids generally have tendency to donate a proton. So, when they are added into the water, hydrochloric acid (HCl) splits into positively charged protons and negatively charged chloride ions. Of this, proton binds itself to the water molecule and forms a hydronium ion (H3O+). Now this hydronium ion is readily looking to pass this proton further to any other molecule, say for example an organic molecule.
But when such organic molecules are forced to accept a proton, it tries to stabilize itself, as most molecules would do. So, it forms a molecule with a double bond – and this is a typical step in the conversion path from biomass to biofuel. Now, zeolite plays an important role here. There are many transition states occurring before the formation of final product. Zeolite walls stabilize these transition states to minimize the total amount of energy required for the conversion to occur.
Zeolites: The acting acids
Zeolite majorly contain oxygen atoms in their crystal structure. This crystal structure of them already contains a proton in them. Like molecular acids discussed earlier (HCl), they also form hydronium ions by interacting with the water molecules. The presence of water creates a suitable ionic framework via the formation of hydrated hydronium ions (H3O+); and negatively charged framework aluminum tetrahedra. However, while hydronium ions remained dispersed in the water, they also remain closely associated to the zeolite present there.

Technical University of Munich (TUM)
(Source: phys.org)
The high density of cationic and anionic pairs determined by the aluminum concentration of zeolite, induces high local ionic strength. This in turn increases the excess chemical potential of sorbed and uncharged organic reactants. Also, chemical pre-treatment can vary the number of active centers and help establish certain density of hydronium ions in the pores of zeolite.
Choosing the ideal Zeolite and Its Endless Applications
The size of cavities was varied, density of active sites was changed symmetrically and the amount of water was varied; which all gave perfect combinations of pore sizes and concentrations of water which could best catalyze the chosen reaction.
Once the perfect Zeolite is chosen to catalyze the conversion; applications are tremendous and in wide range of fields. Biomass (wood, crops, manure, garbage) in past decade has been surged as an important resource for the production of transportation fuels and chemicals. It is primarily based on biochemical transformations like fermentation to produce ethanol from sugar. The conversion of biomass like lignocellulose to biofuels and sugars to chemicals is the most versatile transformation wherein zeolites are used.
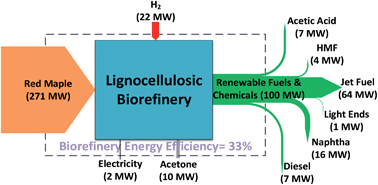
(Source: New Energy And Fuel)
The most important and obvious example is the use of zeolite catalysis in fluid catalytic cracking (FCC) which counts about 45% of the global gasoline stack by converting larger hydrocarbon into gasoline range. Of particulars, other applications include, catalysts for conversion of many oxygen containing compounds, acylation, esterification, oxygenates to hydrocarbons; and many more. Of this, most well-known is conversion of methanol to gasoline (MTG) and ethanol to pyrolysis oil to hydrocarbons used in as gasolines.
Zeolites and the Energy Industry Outlook
Since the discovery of zeolite, the global energy supply has been improved drastically and this allowed production of higher gasoline products from oil than ever before. In 2020, total of 1,100,000 Tons of Zeolite mine production and deposits were reported globally. Of these productions, China, USA, Indonesia and Slovakia accounted for the most abundant deposits around the globe. Zeolites are today an integral role of every oil refinery, whether it be Reliance or Saudi Aramco or Royal Dutch Shell. And, such innovations for the conversion of biomass to biofuels have catalyzed their way for becoming integral part of future bio-refineries too.
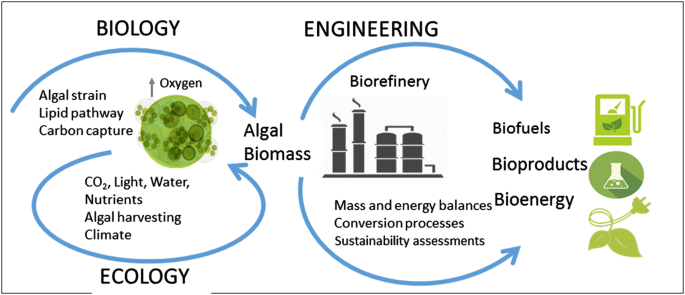
Other significant advances are likely to emerge from new zeotype materials such as stannosilicates (Sn-beta) and titanosilicates (Ts-1, Ti-beta). They have already been potentially demonstrated to be highly active and selective catalysts for the conversion of carbohydrates. They are broadly liked to be applied in the biomass conversion of the future and develop a strong sustainable energy production.
Thus, tailoring of such a molecular environment around the important catalytically active sites has allowed the scientists to explore on the enhancement of reactivity and selectivity of Zeolites through never before scouted pathways.
(Source: thehavok.com)




Biomass is organic matter created by living organisms, which includes plants, animals, agricultural waste products from food production and forestry operations. When heated or smoldered and may be classified as a low-temperature treatment of biomass.