“When heated to the state of steam it is invisible but has enough power to split the earth itself.”
Bruce Lee
Bruce Lee, the most prominent martial artist of all time, once narrated an episode from earlier days when he was a budding martial artist. While sailing through the waters, in his young days, Lee thought of his past training and got mad at himself punched the water! After punching he suddenly realized that water is the essence of kung-fu, which he wished to attain by all sorts of training. Further, he narrated that even after being struck water had no pain and he failed to grab a handful of water. The seemingly weak water that could fit in the smallest of all containers has the strength to shrink the hardest of all things.
With these observations, he found out the inspiring side of the water and its nature. This was a notion of water understood by the apex martial artist while the scientists around the world see to it as “Least understood material on Earth”. Let’s investigate the ice cubes that you pop from ice and see how they are different from the other forms of ice found on earth and other places in the universe.
Ice with Exceptionally Low Density
Water is essentially only the liquid that has a less dense solid form, but this is not the situation with other forms of ice ascertained at diverse places on Earth and stellar space. Ice exists in more than 17 different forms and has created many misapprehension.
Most of the phases of ice form under different conditions of pressure and temperature. Widespread explorations have been conducted to examine the effect of positive pressure which has a predictable result: With the increase in pressure, the density of ice also increases. However, the effects of extreme negative pressure on water molecules are not known. With the aid of molecular dynamics, researchers have theoretically discovered a new class of ice phases and named them aeroice.
This phase of ice has the lowest density among all known ice crystals. With this discovery perception of fundamental properties and behavior of water when restricted to nanotubes and nanopores can be explained.
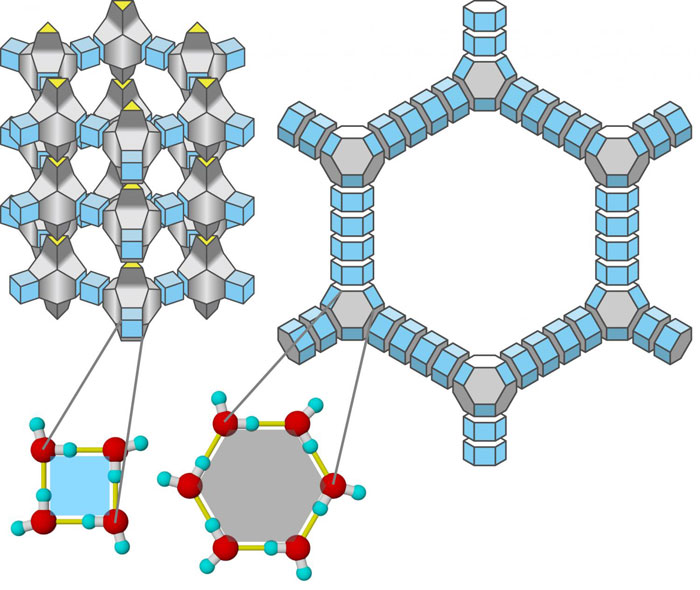
In 2014, the Ice phase that forms under negative pressure was discovered by researchers of Okayama University in Japan. This phase (16) has a 3-D crystalline structure forming a zeolite arrangement. This cage-like structure captures neon in the void space and when the neon is removed it is observed that the molecule is stable and the ice phase has an ultra-low density at extreme negative pressure. Owing to the similarity between the crystal structure if Silica (SiO2) and Ice (H2O ), 300 possible structures were found for which scientists zoomed in through 200 silica zeolites from the database.
The approach can be summarized in a few simple steps: First from the structure of SiO2 oxygen atoms are removed and each Si atom is replaced with an Oxygen atom. The next step of adding hydrogen atoms completes the structure of this ice phase. The density range of this ice is almost half of water or could be close to 0.5 g/cm^3 and among other phases of ice which have zeolite like structure, this is the most stable one.
This research not only interests academic people but also to the scientists who work in different fields. This research can pave the way to an understanding of the behavior of water when confined to nanotubes and nanopores. It would help researchers who aim to colonize other off-world places
One Dimensional Ferroelectric ice
In a new study, a team of chemists has developed a new method for synthesizing a type of ferroelectric ice, which is crystallized so that all of its bonds line up in the same direction, producing a large electric field.
Every water molecule has a diminutive electric field. But the random arrangement of water molecule while freezing causes the electric field to cancel out as the dipoles face in different directions and the ice’s total electric field nullifies. In contradiction to normal ice, the bonds in ferroelectric ice point in the equivalent direction at low enough temperatures, which causes polarization and produces an electric field.
Ferroelectric ice is deemed to be extremely rare. Scientists are still examining whether or not pure three-dimensional ferroelectric ice subsists in nature. Some researchers are working to find evidence of the existence of ferroelectric ice on Uranus and Neptune. Creating pure 3D ferroelectric ice in the laboratory is not feasible, since it would take an estimated 100,000 years to form without the assistance of catalysts. As of now, all ferroelectric ices designed in the laboratory are less than three dimensions and in heterogeneous phases.
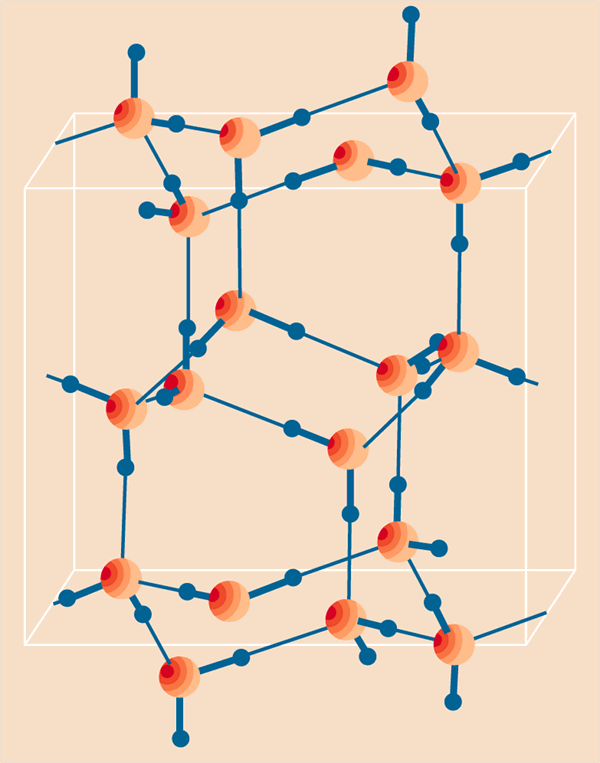
Researchers designed a very thin nanochannel that holds just 96 H20 molecules per unit cell in order to design a water wire. This 1-D arrangement of ice exhibits large dielectric anomalies on lowering the temperature from 350 K to 177 K due to the phase transition. What amazed scientists, even more, is the higher boiling point of nanoconfined water than normal, and still the reason remains unknown.
The hydrogen bonding interaction among the H2O molecules of water wire and nanochannel has affected the ferroelectric property of water. The hydrogen bond remains intact in nanoconfinement and other hydrogen atoms rotate in the influence of the electric field. A property not observed in normal water is that reversing the externally applied electric field polarity of ferroelectric ice also reverses.
Overall, the production of a 1D, single-phase ferroelectric ice using water confined to a nanochannel provides a new way to synthesize ferroelectric materials. The new method could also help scientists better understand the unique properties of ferroelectric ice, which could have applications in the biological sciences, geoscience, and nanoscience.
As Zeng noted, ferroelectric ice could potentially have electrical applications, with the efforts of engineers working in nanotechnology. A new way of synthesizing ferroelectric materials can be devised from this single phase ferroelectric ice using water confined to a nanochannel. With the efforts of engineers working in nanotechnology, ferroelectric ice can have applications in electrical, biological, and geosciences.
Read about black hot form of ice and two different phases of water in our previous post only on thehavok.com




[…] Water is also the strangest liquid on earth. To get more of such interesting content; Click Here. […]
Noice Article bro! Ferro-Electric ice seems to be a wonderful promise for the next generation of Super-conductors.
But the main concern, for now, is the polar ice caps that are melting due to increasing global temperature, resulting in rising sea levels. Many solutions have been proposed to counter this problem. What are your views on this project? (Financially and Environmentally)