Last year, researchers from all over the world fused and fabricated most interesting and marvellous compounds of chemistry. These compounds can be used for betterment of whole world and proved that innovation in chemistry is conducted at ultimate level. Many physicists have said that chemistry is the messy part of physics. But they also believe that, chemistry can be messy to them when they invade it.
This is why, chemistry feels to be a jig-saw puzzle, but at times when these kind of compounds and molecules are involved, people are fascinated. These are compounds which not only fascinate, but make chemistry interesting and innovative. With chemistry like this, future is secured and so is the humankind.
Give a read to most amazing and beautiful synthesis from the previous year’s chemical innovations.
Unbelievable Chemistry: New allotrope of carbon accomplished
Allotropes is a broad concept of chemistry, and unsolved for many elements. Allotropy means that, one element can be existent in different forms based on different arrangement of atoms. For example, carbon has allotropes like graphene, diamond and charcoal.
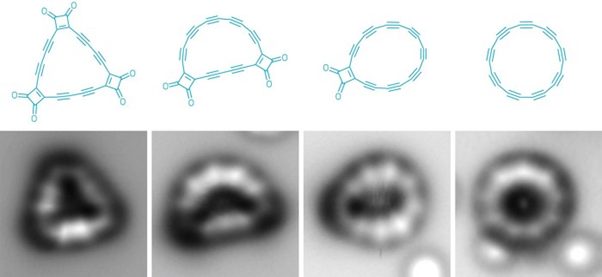
Chemists started with a multi-ring precursor (left) and used voltage pulses to pick off carbon monoxide molecules to form intermediates (center left and right) on the way to making cyclo [18] carbon (right).
The picture above looks hazy, but is taken with atomic force microscope. Researchers made cyclo [18] carbon, which is an 18-membered ring of carbon. This ring is joined together with the help of alternating single and triple bonds.
“It’s both an allotrope and a molecule, which is why this synthesis is so sensational,” says Rik Tykwinski, a physical organic chemist at the University of Alberta
This new allotrope has high reactivity which forms covalent coupling between molecules. Covalent coupling is the formation of single covalent bond between polymeric surface and bio-molecule. small bio-molecules can be immobilized using this. Following this technique, it opens new paths for synthesis of such carbon allotropes and carbon-rich materials.
Anti-aromatic Ni(II) Norcorrole Nano-cage Synthesised:
Since past many decades, molecular cages, hosts and non-porous materials with nano-metre-sized cavities have been reported. Such nano-cages are widely used in molecular recognition, separation, stabilization and promotion of unusual chemical reactions. Also, they are successful drug delivery platforms due to their perfect structures. They are reliable because of properties like bio-compatibility, biodegradable nature and low toxicity.
Majority of these nano-cages or nano-spaces are surrounded by aromatic walls and cages confined by anti-aromatic walls has not yet been synthesised. This is because, instability of anti-aromatic compounds and its unknown effects on properties of nano- spaces.
Although knowing these factors, researchers still demonstrated the construction of anti -aromatic-walled nano-cage which is self-assembled nano-cage composed of four metal ions with six identical anti-aromatic walls.
This was made possible, as they made these Ni(II) Norcorrole building blocks and added substituents and iron ions to adjust according to conditions and molecule self-assembled itself into tetrahedral shape.
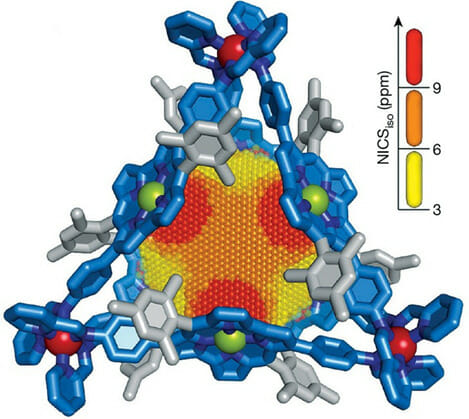
Molecules that land inside this nano-cage have their nuclear magnetic resonance (NMR) signals shifted downfield, depending on their location, 3 ppm (yellow) to 9 ppm (red). Blue sticks represent anti-aromatic walls; gray represents substituents on walls. Ni = green; Fe = red are substituents.
This cage showed unusual behavior beyond the NMR-spectroscopic frequency range. This can open new ways for further study of effects of an anti-aromatic environment on nano-space.
Longest, most twisted Perphenylacene (per-phenyl-acene) in chemistry synthesised:
Dodeca-phenyl-tetracene, is the largest prepared Perphenylacene and was synthesized from known compounds in three steps.
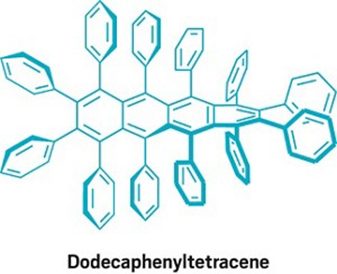
From the figure It can be seen that, there are main 4 fused benzene rings in the centre. These are surrounded by 12 pendant phenyl rings and makes up the whole structure. Because of such surrounded rings, it becomes almost nonreactive. Thus, it displays reversible electrochemical oxidation and reduction reactions.
This compound is of high interest for electronics and photovoltaics due to its significant chemical and physical properties.
Chemists cage methane inside C60 fullerene:
Fullerenes or buckyballs, are the roundest molecules known in chemistry. They are allotropes of carbon. Fullerenes, are versatile and have antiviral properties. Because of this, they are used in the treatment of HIV-infection. Methane is the largest and first organic molecule to be encaged inside a Buckyball.
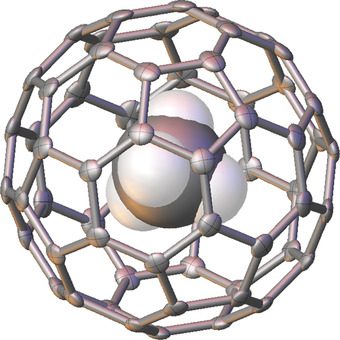
Researchers synthesised the fullerene cage with a sulfur containing 17-membered ring, forced methane inside at high pressure, and closed the cage by oxidizing the sulfur. The remaining sulfur monoxide was ejected later. This whole synthesis was characterized with the help of mass spectroscopy, NMR spectroscopy and X-ray crystallography. After the synthesis, there were evidences of methane freely rotating inside the C60 cage.
This synthesis, opens opportunity of encaging even larger molecules like, NO, NH3, N2, CO2, CH3OH and H2CO. With this, new possibilities in the field of nanotechnology and nano-chemistry can be flourished.
Chloride ion capturing Cryptand-Cage for chemistry:
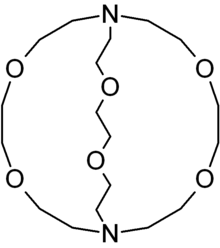
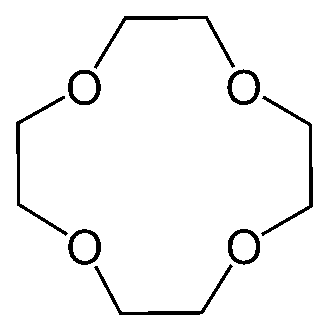
First of all, lets get familiar with cryptand. These are basically 3-D forms of crown ethers with more selectivity and are strong. So, now what are crown ethers? They are nothing but cyclic chemical compounds possessing ring with multiple ethers. This is structure of a cryptand.
Now, knowing these we can move further. Any bio-molecular structure or compound have synthetic receptors which are associated with O-H or N-H hydrogen bonding. This give them high selectivity and tight binding. But, contrary to this, researchers developed a chloride-selective receptor in the form of cryptand-like cage. This too, they have done with only C-H hydrogen bonding, which are considered weak hydrogen doners and offer weak hydrogen bonding.
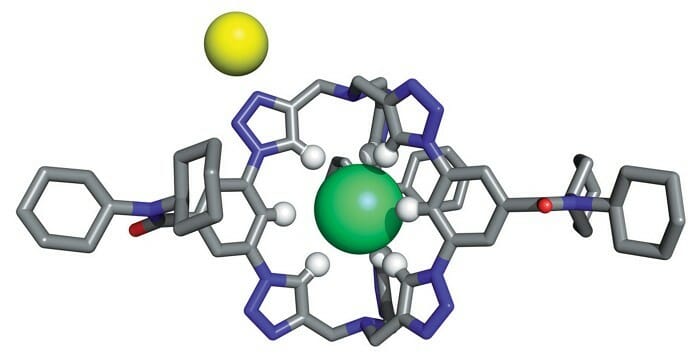
This compound synthesised has a corrosion inhibition property. It means that, it can slow down the corrosion rate of metal or alloy that comes in contact with fluid. Also, it shows anti-Hofmeister salt extraction. This effect in simple terms is related to the solubility of proteins. (click here to know more)
Catenanes- The interlocked rings of benzene
Nanotechnology and especially nano-carbons are boom to the field of science and technology. Researchers at Japan have developed this dynamic topological molecular nano-carbons, and stimulated its deeper understanding and applications. They have composed catenate and trefoil structures exclusively from carbon and hydrogen.
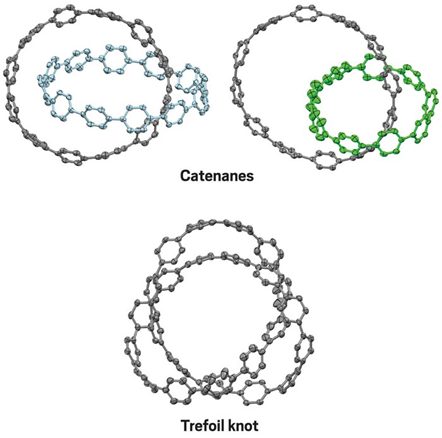
They here linked different phenyl rings end to end into macro-cycles. These macro-cycles met at silicon centers in the middle. After this was achieved, they removed the silicon with fluoride, and final products were achieved. This catenanes seem to be rigid in nature. But it was observed that these all-benzene ring structures possessed rapid vortex-like motion even at -950C. Thus, this was interesting dynamic behavior from such a molecular compound and extended deeper study of such topological molecular nanocarobons.
This was all about the coolest chemistry witnessed from last year. Still, putting up all of the research work here was not feasible and is tiring. Mention one of your favourites from the above exciting compounds you want to know more about in the comment section below. With ideas and research, we will surely try to put up the compound of your interest.
Do comment for your favourite.
with such chemistry, achievements become frenzy, require hard work of many
and indeed trails whole science with excellency.
–OSD




[…] research also extends the opportunity of exploring what is beyond the edge of the periodic table, and possibly discovering a new element. With more such findings we have found ourselves in a […]