2020 has been full of surprises that no one would have ever asked for. From Wildfires, worldwide disease spread followed by economic hardships, lockdowns, mental traumas to vaccines, most of us made it through the end of this so-called year of staying alive. People also seemed to be experiencing loses from the productivity viewpoint, however, the scientific community, especially from chemical sciences background, had an uncommon time in laboratories and came up with striking discoveries with the essence of innovativeness.
These days people seem fed up of headlines that create negative sentiments, again owing to the downfall in mental health, like that of economies falling, shutdowns, second waves and geopolitical conspiracy tales behind pandemic and other so-called evil theories. Having said that, this happens to be a good occasion to get acquainted with researches with the pure essence of chemical sciences. These innovations could lay the foundation of sustainable, green, plastic-free and undoubtedly, a brighter age of humanity. Let us look at what else, other than vaccines, chemical sciences has decided to give away as new year present:
New Nitrogen-Assembly Carbon catalyst: Reshaping the Chemical Manufacturing Methods
Scientists at the U.S. Department of Energy’s Ames Laboratory have devised a metal-free carbon-based catalyst that has the ability to be more efficient for many industrial applications, including the production of bio- and fossil fuels, electrocatalysis, and fuel cells.
In a general sense, these industrial processes include splitting strong chemical bonds, hydrogen-hydrogen, carbon-oxygen, and carbon-hydrogen bonds to name a few. Traditional ways of accomplishing such bond splittings are by using catalysts that use transition elements or precious metals, most of them are expensive and low in natural abundances, platinum and palladium, for instance.

The scientists conducted experiments with a type of heterogeneous catalyst, Nitrogen-Assembly Carbons (NACs), in which the design and placement of nitrogen on the carbon surface greatly influenced the catalytic activity of the material. These N atoms on carbon surfaces were previously believed to be distant from one another, as the close placement of N atoms is thermodynamically unstable.
Scientists derived and conducted experiments on a new type of heterogeneous catalyst, Nitrogen-Assembly Carbons(NACs). The catalytic activity of this catalyst is influenced by the design and placement of nitrogen atoms on the surface of the carbon. Owing to the thermodynamic instability of closed placing of Nitrogen atoms, it was assumed that the N atoms are arranged distantly.
The team of scholars at Ames Laboratory also correlated the N precursors and the pyrolysis temperature for sysnthesis of the NACs, and discovered that unexpected catalytic reactions were delivered by the meta-stable assembly of Nitrogen atoms that were designed separately.
Reactions like dehydrogenation of ethylbenzene, hydrogenolysis of aryl ethers and the most common among all hydrogenation of unsaturated functional species containing keto, alkene, alkyne and nitro groups. Further, this catalyst is robust and consistent in delivery products from the both gas phase and liquid phase reaction mixture under extreme condition of temperature and pressure.
Chemically driven wheels that transform to gears and perform mechanical work
Gear being one of the oldest mechanical tools in industrial history, it has led the development of machines ranging from clocks and irrigation tools to high-end robotics and Modern engines. Researchers from the University of Pittsburgh came up with a method of utilizing a catalytic reaction that pushes a chemically coated, two-dimensional sheet to spontaneously transform into a three-dimensional gear that performs mechanical work.
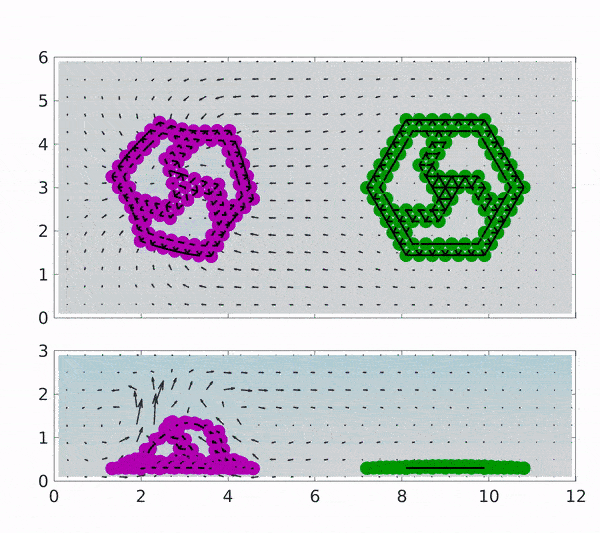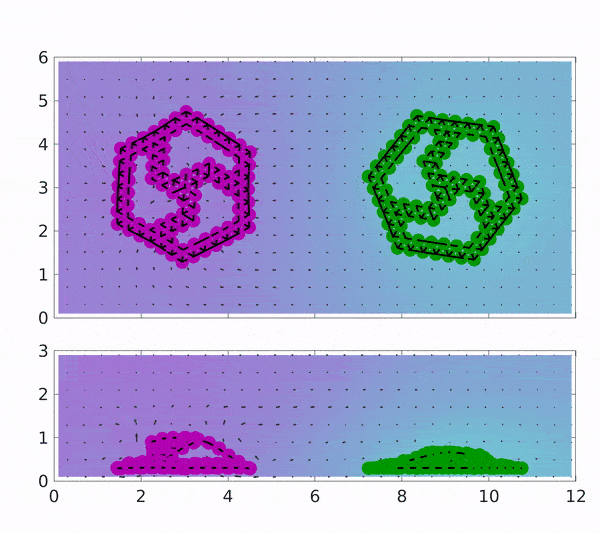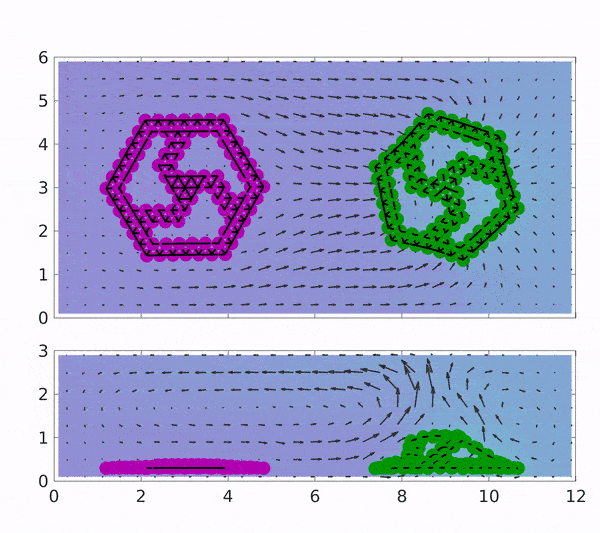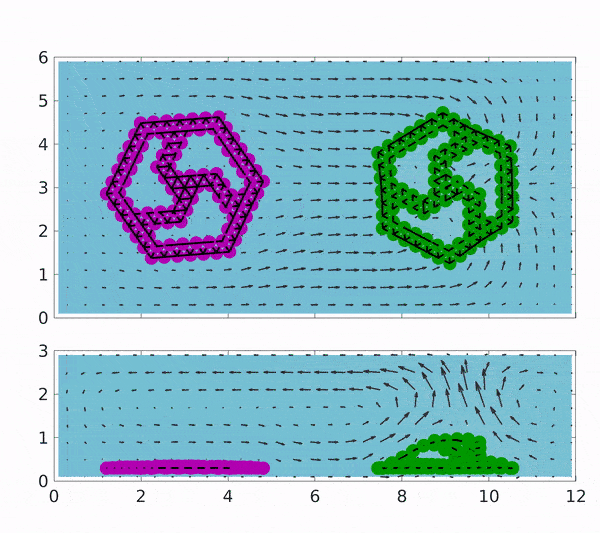
These findings could pave the way towards industries based on chemically driven machines and minimize the need for external power by depending on a chemical process involving the addition of the reactants to the surrounding solution. The motion of both magenta coloured rotor which is coated with Graphene oxide(GOx) and of green coated with CAT is placed in a solution containing H2O2 indicated by spatial background map.
The first reaction that occurs produces H2O2, which makes up the first step of the cascading reaction. Due to the presence of H2O2, the motion of the CAT coated rotor is triggered, while the GOx rotor converts to 2 D and stops rotating due to the depletion of glucose in the solution. Gradually, the H2O2 is also exhausted and the sheets become flat with no motion at all but can be started upon the addition of reactants.
Balazs expressed his views about this innovation: “The more remote a machine is from human control, the more you need the machine itself to provide control in order to complete a given task, The chemo-mechanical nature of our devices allows that to happen without any external power source.”
These self-morphing gears are the latest evolution of chemo-mechanical processes developed by Balazs, Laskar, and Shklyaev and are foreseen to be used for large scale machinery, however, this could be used directly to drive some reactions in soft robotics. With this, chemical sciences seem to have many more surprises hidden in the fume hoods of labs.
Platics Alternatives Derived from Industrial Waste

Research Scholars at one of the Brazilian University have developed a film made from scraps left after industrial p processing. The raw materials used are sustainable and can be used for food packaging. This finding clears the path towards a plastic-free lifestyle of humanity.
The film is made from hydroxypropyl methylcellulose (HPMC) and bacterial cellulose and it outperformed the film made only using HPMC. They filled the HPMC matrix with bacterial cellulose nanocrystals to improve it’s properties and also to create a green protocol for the development of new-age composites by including the reuse of industrial waste.
A limitation of the films made of HPMC and other biopolymers is their small range of mechanical strength when compared with those derived from petroleum products. High permeability to water has also limited their application. However, these shortcomings were improved by introducing bacterial cellulose.
Among several advantages, the one that stands apart is the presence of nanometric fibres in its structure, as this gives the material can withstand certain loads and stresses without cleaving. The use of cellulose scraps and similar wastes can help in diminishing the processing cost.
The use of cellulose scraps and other kinds of waste also helps reduce processing cost. Consumers typically prefer plastic film for packaging because it is cheap. Moreover, there are other promising sources of bacterial cellulose, such as the sugarcane and soy industries.
More such researches in the field of chemical sciences have not only awakened the hopes for more sustainable human lifestyle but also helped in tracing the path towards making planet Earth, a safer place for all life forms.
Chemical Sciences




I’ve been exploring for a little bit for any high-quality articles or weblog posts in this kind of house
. Exploring in Yahoo I eventually stumbled upon this web site.
Studying this information So i am happy to express that I’ve a
very good uncanny feeling I came upon just what
I needed. I such a lot definitely will make sure to don?t forget this web site and give it a look
on a continuing basis.
Thanks for sharing your thoughts on website. Regards
Very nice post and right to the point. I dont know if this is truly the best place to ask but do you folks have any ideea where to employ some professional writers? Thank you
I absolutely love your blog and find most of your post’s to
be just what I’m looking for. Do you offer guest writers to
write content available for you? I wouldn’t mind
composing a post or elaborating on some of the
subjects you write regarding here. Again, awesome site!
Surely Rodney. We would love to have you as a guest author. You can share your work to thehavok.com on its official G-mail iD – thehavokdotcom@gmail.com
We will review the article and let you know.
Thanks.
thehavok.com