“Carbon dioxide” this word brings images of pollution, industrial waste, climate change, global warming to our mind, but we are at the beginning of an era where we will have a different perception of the ongoing energy crisis, all thanks to the scientific innovations in the field of energy sciences and engineering. From air capturing technology to CO2 recycling, many such advancements are on their way to bring about a revolution and overthrow the traditional energy sources.
Utilizing the excess carbon dioxide which is a by-product of major industrial processes could do wonders if not simply released or disposed at the ocean beds. Several research projects are being undertaken to develop an effective technique to direct CO2 emissions towards various energy storage based projects.
Following are some cutting edge projects and discoveries that deploy not so eco-friendly carbon dioxide gas:
Ethanol synthesis using Copper based electro-catalyst
Scientists from Argonne National Laboratory claim to have build a catalyst that recycles CO2 into energy-rich ethanol. Further, this process can be powered by renewable energy. This method has an efficiency of nearly 90% which is more than any other industrial process known. Synthesis of ethanol is just one process out of the possible long list of recycling CO2 into other useful chemicals.
Breaking chemically stubborn carbon dioxide at low energy cost can pave the way to multiple ways of producing useful chemicals and can start a domino effect of new discoveries in the field of energy sciences. The most immediate opportunity that would open up is converting CO2 into hydrocarbons. These processes can be so efficient that the mitigation of CO2 at the bedrock will have to find its way out.
What makes this catalyst so magical is its composition. The catalyst consists of atomically dispersed copper on carbon-powder support. Under an external electric field, an electrochemical reaction is initiated in which catalyst breaks down CO2 and H2O molecules to selectively reassemble the broken molecule into ethanol. The electrocatalytic efficiency (Faradaic efficiency) is close to 90%.
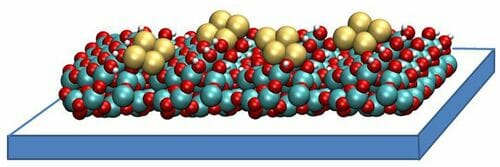
The research was benefitted from the facilities like the Advanced Photon Source (APS) and Centre for nanomaterials (CNM) which has the Laboratory Computing Resource Center (LCRC). The high photon beam at APS helped in detecting the structural changes in the catalyst during the electrochemical reaction. A high-resolution electron microscope at CNM and modeling at LCRC revealed the reversible transformation between atomically dispersed copper and clusters of three copper atoms each. This finding sheds light on further improving the catalyst by deploying rational design.
Scientists at ANL, now look forward to deriving new catalysts through a similar approach to convert CO2 into hydrocarbons. It has the potential to bring major reforms to the industrial world.
Direct Air Capture Technology
/cdn.vox-cdn.com/uploads/chorus_image/image/60061703/152822801433752053_cropped_for_web.0.png)
What if we could extract CO2 from the atmosphere just like plants and trees. The answer is, we could have a solution to tackle climate change and bring about energy reforms. Industries, vehicles, power generators, and other human needs consume fuels to deliver the desired outcome and in turn release CO2 and other harmful gases. Scientists and industrialists took this environmental issue as an opportunity to stride towards carbon neutrality. Direct air capture technology is something that has been developed to create a loop to produce fuel out of CO2
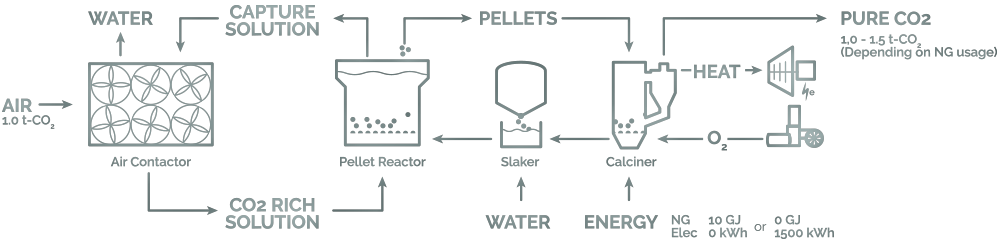
Carbon Engineering is a company located in British Columbia that has a CO2 sucking and processing plant located between high cliffs and valleys. This plant uses giant fans (air contactor) present under the open sky driven by renewable energy sources to suck the air directly from the atmosphere. Pulled air then becomes a part of a series of chemical reactions to extract CO2 and release other gases back to the atmosphere.
Polymer sheets are placed such that air passes through the dripping potassium hydroxide. Again, this chemical solution has low toxicity and by exploiting the acidic nature of CO2, corresponding carbonate salt is obtained in the form of pellets which on heating release CO2 and remaining part of the salt is hydrated and returned to the initial form. Further, carbon dioxide collected is used in food processing or combined with hydrogen to obtain hydrocarbons and sold as synthetic oil in markets.
On a large scale, capturing a ton of CO2 costs them US$100. CE uses a combination of natural gas and renewable electricity to power its plant. CO2 emissions of natural gas are also directed to the system for capturing CO2. Their plant is capable of relying completely on renewable electricity but engaging it in a combination with natural gas cuts down the costs. Clearly, capturing CO2 has got zero-emission, in fact, a larger amount of it is being taken from the environment and is stored for further actions.
Being economically backed by Bill Gates, Chevron, and Occidental, CE aims to scale up and install plants in other parts of the world. However, not everyone approves this as a solution to tackle climate change or reduce CO2 emissions, professors from the University of Calfornia and Stanford University see partnering with oil companies is a wrong step and all this would promote fossil fuel usage instead of working in the direction of renewables. Investments in renewable energy harnessing projects like solar and wind energy could help more, environmentally, as these do not have the emissions in the first place.
What these projects do is not so complex and do not require traditional energy sources to operate on large scales. Each year the world emits about 40 billion tons of CO2, at this rate, it would take 40,000 such plants each capturing 1 megaton of CO2 per year. Many other companies based in different countries have already begun commercial air capturing. Political and technical scenarios associated with the direct air capture technology point towards a cleaner and greener future.
Lithium-Carbon Dioxide Battery

Lithium-ion batteries have found their use in multiple domains. From electric vehicles to mobiles, laptops, and other handheld devices, all run on lithium-ion batteries, and what makes it so ubiquitous is its energy density and recharging frequency. Energy sciences is a domain that has thousands of people engaged to find the most suitable devices that have capabilities to meet ever-increasing energy needs.
Researchers at the University of Illinois, Chicago have tested a design of lithium-carbon dioxide battery prototype which can be recharged completely and run efficiently even after 500 consecutive cycles of charge-recharge process.
Read further about Lithium-Carbon dioxide battery and new innovations in here.




[…] typically encompasses two very different conceptions: First is Carbon Capture, sucking CO2 out of the air so that less heat is trapped. The second is blocking excess sunlight […]
[…] the set-up is that it is closed. The aforementioned makes it much more easygoing and cheaper to capture CO2 emissions. These can then be used as an ingredient for the chemical industry. To filter out […]
[…] Read the previous blog Carbon dioxide: Can a greengouse gas meet energy need […]
Can’t wait to read more about Li-Carbon dioxide battery.
What if the commercially developed carbon capture tech is made mandatory for all companies producing Carbon dioxide above a certain threshold limit?